| STATE CHART OF TIN/LEAD ALLOYS |
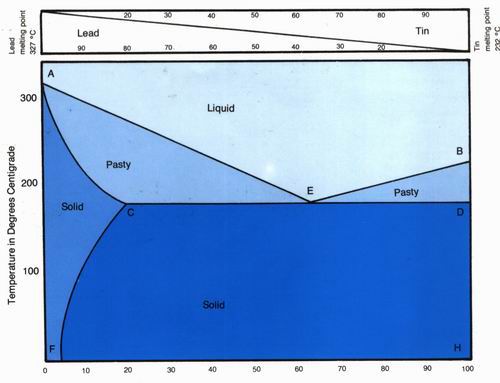
The interpretation of the state chart of
alloys is of outstanding importance in metallurgy, as it is possi ble to forsee
the structure and the properties, above ali mechanical, of an alloy obtained by
cooling a fusion of two or more metals in a known percentage.
Soldering alloys
consist of Tin and Lead
Tin melts at 232 °C and lead at 327 'C.
Tin/lead
alloys, whose characteristic is defined by the content of tin, are in fact
commonly called "sol derings"; they have the property of melting at
temperatures lower than those of tin/lead's fusion. On the abscissas of the
shown chart are reported the composition in weight, and, on the ordinates the
corresponding temperatures, as well as the melting point of the respective
alloys which can be realised.
Each point in the chart represent therefore an
alloy heated at a definitive temperature.
Of great importance is the eutectic
composition E: it has a lower melting temperature, not only of the singie
chemical species of which it is formed, but also of any other mixture of them.
Even if it ha e well defined composition and fusion temperature, it isn't a
chemical compound, as, when it is in the solid state, it is possible to
distinguish the chrystal of both components by means of a microscope The
eutectic composition E, which consists of 63% tin and 37% lead, is the one
which is mainly taken into consideration for the employements stricly connected
to the electronic field, just for its charac teristic of having the lowest
melting point.